🔊Welcome to the next lesson in the series of Biochemistry basics. In today's lesson will discuss oxidative phosphorylation better known as electron transport chain. By the end of this lesson, you will be able to understand the process of ETC, how electrons are shuffled through the chain, how ATP synthesis works using a proton gradient, and how electron chain inhibitors inhabit various parts of the electron transport chain. And you will also come to know about the total ATP production in all of these biochemical pathways.
👉Technically the whole structure is broken into two distinct parts of oxidative phosphorylation. Complex I to IV including CoQ and cyt C; are included in the respiratory chain because the electrons are shuffled from one complex to the other in this chain along the gradient.
The ATP synthase which pumps protons to generate ATP is technically known as chemiosmosis.
👉The combination of the electron transport chain and chemiosmosis together is known as oxidative phosphorylation.
Location:
Goal :
Components of ETC:
In the electron transport chain, the electrons are transferred from NADH to a chain of electron carriers. The electrons flow from the more electronegative components to the more electropositive components.
All the components of the electron transport chain are located in the inner membrane of the Mitochondria. The four complexes are connected by two mobile careers, coenzyme Q, and cytochrome C.
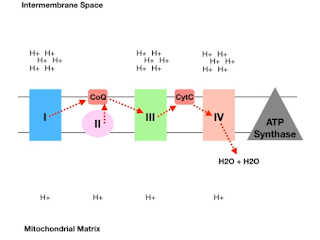
The sequence of reactions is shown in the figure.
The four complexes are:
- 1. Complex I (NADH Dehydrogenase)
- 2. Complex II (Succinate Dehydrogenase)
- 3. Complex III (Cytochrome b oxidoreductase)
- 4. Complex IV (Cytochrome oxidase) and
- Electron Carriers CoQ and CytC
Understanding how the complexes work;
Complex I :
So the process starts with the entrance of NADH (from TCA cycle) and it approaches Complex I, NADH can give up its proton and electron to become NADH +; in this process, it donates its electrons to Complex I and complex I become supercharged. When this Complex gets supercharged it has the energy to pump the protons from intermembrane space as it pumps more and more protons you get the accumulation of protons on the outer side of the membrane. This pumping is only made possible by the donation of the electrons from NADH to Complex I.
After a while, the electrons will set in complex I, and a strong Proton gradient will begin to form. The protons in the intermembrane space(top) will be more than and the mitochondrial matrix(bottom). Complex I will pass electrons to CoQ and electrons will sit there and wait for further instructions.
Complex II:
In the 2nd phase FADH2 will approach Complex II and begin to give up electrons by converting into FA D. ComplexII, however, cannot become supercharged as there is not sufficient energy. So the electrons will sit in complex II and start to accumulate there, ultimately are passed on to Co Q.
✋Points to be noted are:-
- NADH can pass electrons to Complex I and
- FADH2 can pass electrons to complex II.
👉So
- THE electrons from NADH go from Complex I to coenzyme Q and
- The electrons from FADH2 go from Complex II to coenzyme Q.
It is important to understand that coenzyme Q is the final electron acceptor for both Complex I and complex II.
👉It is also important to remember that
- NADH can give electrons only to Complex I and
- FADH2 can give electrons only to Complex II.
Complex III :
All the electrons from coenzyme Q are passed to Complex III. when the electrons go from coenzyme Q to Complex III it is supercharged, and there is enough energy released to create a potential gradient to pump protons from the matrix through Complex III, into the intermembrane space (just like Complex I).
Complex IV:
In the intermembrane space, there is a much greater positive charge than the Matrix. So a very big Proton gradient is being formed. Complex III will now pass electrons to cytochrome C and cytochrome C will pass these electrons to Complex IV, making it supercharged and creating enough energy to pump protons from Matrix to the intermembrane space(just like Complex I and complex III).
At this point is complex IV has enough electrons sitting inside it and these electrons need to pass on to some electron acceptor. Oxygen is the final electron acceptor. At this point, it takes electrons and gets split into two oxygen ions and two protons(H+) are added to create two molecules of water.
✋The point here to be noted is that in the electron transport chain(ETC) the final electron acceptor is Oxygen and by accepting electrons and protons it creates two molecules of water.
👉Note:
By looking at the electron transport chain it is clear that
- 1. Complex 1 and complex II, pass electrons to coenzyme Q;
- 2. Coenzyme Q passes the electrons to ComplexIII;
- 3. Complex III then passes the electrons to cytochrome C
- 4. Cytochrome C passes electrons to Complex IV and
- 5. Complex IV gives the electrons to the ultimate electron acceptor oxygen.
- 6. Oxygen split into oxygen ions and with the addition of protons, it produces two water molecules.
✋A quick recall: at this point, it is clear that electrons shuffle from one complex to the next and in this process, they supercharge Complex I, III, and IV so that these complexes can pump protons from Matrix to intermembrane space.
ATP Synthase:
Fig 3: formation of ATP from ADP
At this point intermembrane space is becoming so full of protons as compared to the matrix hence there is an energy gradient available to be used. Now the ATP synthase comes into play; it uses the proton gradient to generate ATP.
To convert low-energy ADP to high-energy ATP, we need an energy source, and this energy source is the proton gradient.
👉Remember: Protons always want to move down the gradient, that is, from high energy gradient to low energy gradient to acquire more stability or equilibrium.
So the protons will flow from intermembrane space to the matrix to achieve more stability when they do so the energy produced is used to catalyze ADP into high-energy ATP and a massive amount of ATPs are produced.
It is a Cyclic Process:
The protons in the matrix keep on accumulating as long as NADH and FADH2 are being shuffled from TCA to etc and they keep on supercharging complexes I, III, and IV.and the whole process will keep on repeating itself as long as the proton gradient is maintained.
Inhibitors of oxidative phosphorylation:
- 1. Rotenone inhibits Complex I.
- 2. Antimycin inhibits Complex III
- 3. Cyanide and Carbon monoxide inhibits Complex IV and Cytochrome C
- 4. Oligomycin inhibits ATP synthase
- 5. Uncoupling agents and uncouple the proton gradient and hence inhabit its ability to pump protons from intermembrane space to matrix
ATP yield:
The purpose of ETC is to generate ATP and total ATP generation from glycolysis, gluconeogenesis, pyruvate metabolism, and TCA cycle along with oxidative phosphorylation are shown in the table below, total ATP yield is very high and it comes around to 32 ATP.







0 Comments